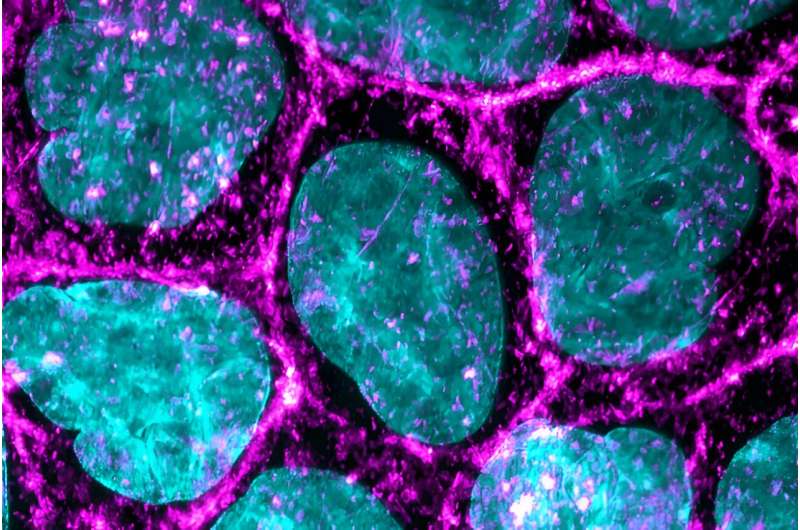
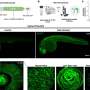
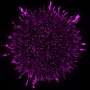
Microscopy image of the epithelial cells used in the study, the cytoskeleton (magenta) and nuclei (cyan) of the cell are fluorescently labeled, allowing the detailed studies of the structures. Credit: Heidi Peussa
Life sciences and photonics researchers at Tampere University have made a remarkable discovery in studying superficial cells' response to mechanical stimuli. By simulating the deformation of the extracellular matrix below the cells, researchers have shown that the cells quickly sense even minor changes in their environment, and their response is more complex than expected. The discovery may help to better understand, for example, the processes related to cancer metastasis formation.
Three research groups investigated in this joint project how epithelial cells sense small changes in their environment through ion channels. The study was conducted using light-responsive materials developed by the Smart Photonics Materials research group led by Professor Arri Priimägi, which can be used as a substrate for cell culturing. These materials allow precise and controllable movement of the cell substrate using light stimulation.
"The cells had a marker protein for intracellular calcium, so we were able to draw small grooves on the substrate surface on a confocal microscope and at the same time monitor how living cells respond to these changes in the environment with the help of calcium," says Teemu Ihalainen, Senior Research Fellow at Tampere Institute for Advanced Study (IAS) and leader of the Cellular Biophysics research group at the Faculty of Medicine and Health Technology.
"We found that even the movement of a few tens of nanometers of material opened mechanically gated calcium channels in the cells, through which the cells were able to change their calcium levels."
Calcium is needed in cells for a wide variety of processes, so even small changes in the amount of calcium can have large effects on cellular functions. The study shows, perhaps for the first time, that cells are able to sense minute movements in their environment and these movements are detected by changing the flow of calcium ions through the cell membrane, i.e. electrically via ionic currents.
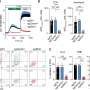
The study focused on intracellular calcium changes during the first seconds of the mechanical stimulus. An article titled "Light-induced nanoscale deformation in azobenzene thin film triggers rapid intracellular Ca2+ increase via mechanosensitive cation channels," which is a key part of Doctoral Researcher Heidi Peussa's dissertation, was published in the journal Advanced Science.
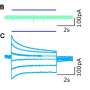
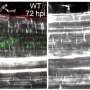
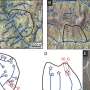
Calcium indicator expressing epithelial cells were grown on Dispersive Red 1 (DR1)-glass substrate. The substrate deformation and topographical change was induced by generating line-like patterns to the surface with 488 nm laser light. The rise in image brightness (before vs. after) indicates rapidly increasing calcium concentration in the cells. Credit: Heidi Peussa, Tampere University
Mechanically gated ion channel as a key
In the body, epithelial cells are tightly attached to the extracellular matrix, allowing mechanical strain of the environment, for example, to be transmitted to the cells. Mechanical stimuli are important in the normal functions of cells. Disruption of cell attachment often causes disease or other problems.
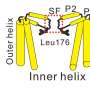
Cells sense changes in their environment in a variety of ways, for example, by mechanically gated PIEZO1 ion channels. The channels can be understood as cell membrane pores that are closed in a mechanically relaxed state, but open as the cell membrane stretches. The opening happens in thousandths of a second and leads to calcium influx into the cell. The process has a key role in many physiological functions, e.g., in touch sensation. The discovery of mechanically gated ion channels was awarded the Nobel Prize in 2021.
More information: Heidi Peussa et al, Light‐Induced Nanoscale Deformation in Azobenzene Thin Film Triggers Rapid Intracellular Ca2+ Increase via Mechanosensitive Cation Channels, Advanced Science (2023). DOI: 10.1002/advs.202206190
Journal information: Advanced Science
Provided by Tampere University