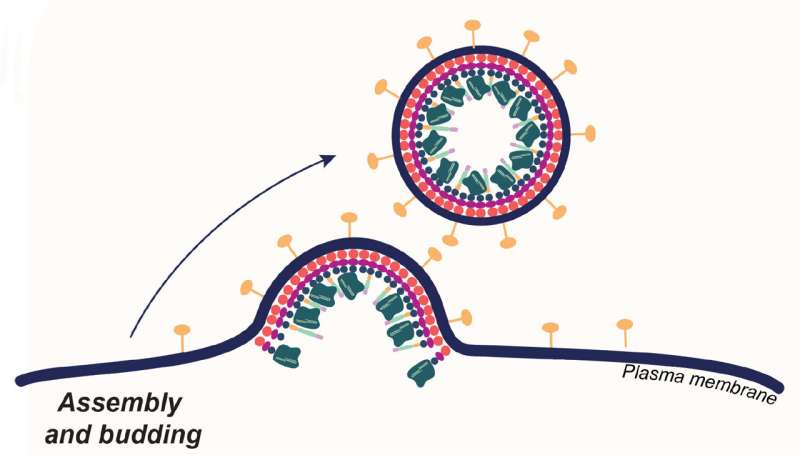
Enveloped viruses get their outer coat by budding from cells they've invaded. CRISPR-Cas9 researchers coopted this behavior to produce envelope-derived vehicles that encapsulate Cas9 proteins (dark green), guide RNA and transgenes. These loaded carriers target and invade specific types of human T-cells, where they simultaneously edit and insert new genes, turning the T-cells into cancer fighters. Credit: Jenny Hamilton, IGI/UC Berkeley
Most approved gene therapies today, including those involving CRISPR-Cas9, work their magic on cells removed from the body, after which the edited cells are returned to the patient.
This technique is ideal for targeting blood cells and is currently the method employed in newly approved CRISPR gene therapies for blood diseases like sickle cell anemia, in which edited blood cells are reinfused in patients after their bone marrow has been destroyed by chemotherapy.
A new, precision-targeted delivery method for CRISPR-Cas9, published in the journal Nature Biotechnology, enables gene editing on very specific subsets of cells while still in the body—a step toward a programmable delivery method that would eliminate the need to obliterate patients' bone marrow and immune system before giving them edited blood cells.
The delivery method, developed in the University of California, Berkeley, laboratory of Jennifer Doudna, co-inventor of CRISPR-Cas9 genome editing, involves wrapping the Cas9 editing proteins and guide RNAs in a membrane bubble that has been decorated with pieces of monoclonal antibodies that home in on specific types of blood cells.
As a demonstration, Jennifer Hamilton, a CRISPR researcher in the Doudna laboratory at the Innovative Genomics Institute (IGI), targeted a cell of the immune system—a T-cell—which is the starting point for a revolutionary cancer treatment called chimeric antigen receptor (CAR) T-cell therapy.
Hamilton and her colleagues treated live mice that had been equipped with a humanized immune system and turned their human T-cells into CAR T-cells able to home in on and eliminate another class of immune cell, a B cell.
The feat was a proof of principle, Hamilton said, showing the potential to use this carrier method—enveloped delivery vehicles—to target and edit blood cells and potentially other types of cells in living animals (in vivo) and, eventually, humans.
"Our approach involves multiplexing targeting molecules, that is, having two or more targeting molecules on our particles that interact with their target cell somewhat like an AND gate in a computer," said Hamilton, referring to logic circuits that operate only when two events happen simultaneously.
"We were able to get more effective delivery when the particles bound using two antibody ligand interactions. After treating mice with T-cell-targeted vectors, we observed genome engineering in our cell type of interest, T-cells, and not in liver hepatocytes."
Highly specific targeting is difficult for all methods of delivering genes into cells, she said. Liver cells, in particular, often take up delivery vehicles directed elsewhere.
Viral envelopes
Hamilton and her team are investigating one of several experimental techniques for delivering gene therapies. Many employ the outer coat of encapsulated viruses—the viruses are emptied out and stuffed with corrective transgenes or gene editing tools such as CRISPR-Cas9.
More information: Jennifer R. Hamilton et al, In vivo human T cell engineering with enveloped delivery vehicles, Nature Biotechnology (2024). DOI: 10.1038/s41587-023-02085-z
Journal information: Science , Nature Biotechnology
Provided by University of California - Berkeley